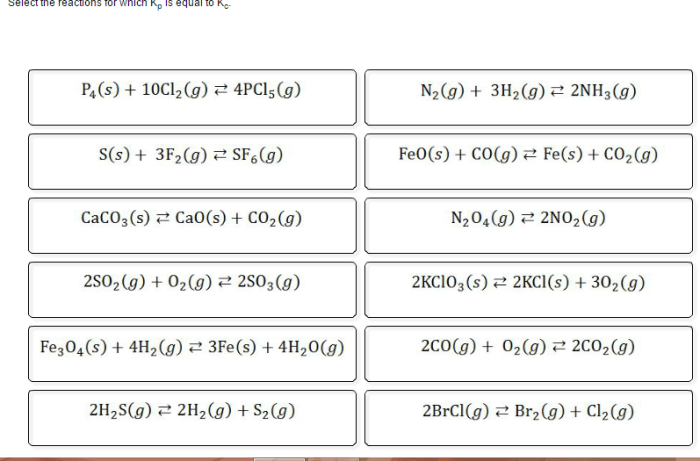
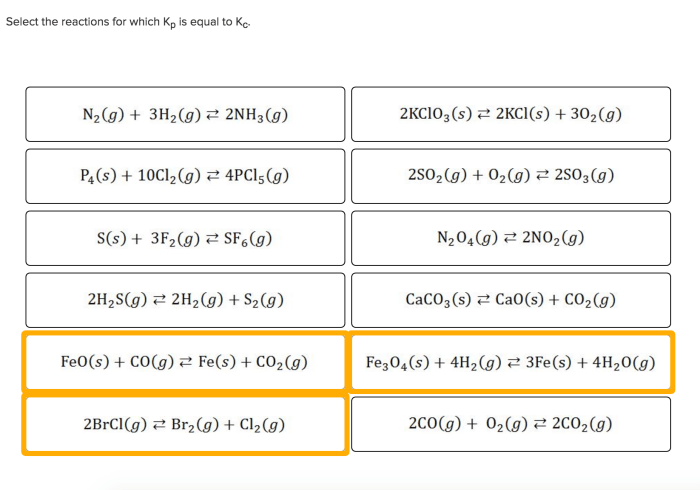
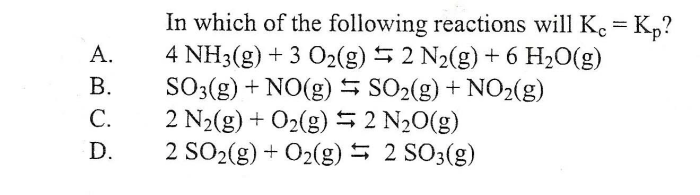Select the reactions for which Kp is equal to Kc, a fundamental concept in chemistry that unveils the intricate relationship between equilibrium constants in gas and solution phases. This exploration delves into the factors influencing these constants, their practical applications, and the underlying assumptions that govern their equality.
Equilibrium constants, Kp and Kc, quantify the extent to which chemical reactions proceed towards completion. Understanding their interplay is crucial for predicting reaction outcomes and manipulating chemical systems.
Understanding Equilibrium Constants
Equilibrium constants (Kp and Kc) are quantitative measures of the extent to which a chemical reaction proceeds towards completion. Kp is the equilibrium constant expressed in terms of partial pressures, while Kc is expressed in terms of molar concentrations.
The relationship between Kp and Kc is given by the equation:
Kp = Kc(RT)^(Δn)
where R is the gas constant, T is the temperature, and Δn is the change in the number of moles of gas during the reaction.
For example, consider the reaction:
N2(g) + 3H2(g) ⇌ 2NH3(g)
The equilibrium constant for this reaction is:
Kp = (PNH3)^2 / (PN2)(PH2)^3
and the equilibrium constant in terms of concentrations is:
Kc = [NH3]^2 / [N2][H2]^3
Factors Affecting Kp and Kc
The values of Kp and Kc can be affected by several factors, including:
- Temperature:Increasing temperature generally increases the value of Kp and Kc for exothermic reactions (reactions that release heat) and decreases the value of Kp and Kc for endothermic reactions (reactions that absorb heat).
- Pressure:Increasing pressure generally increases the value of Kp and decreases the value of Kc for reactions involving gases.
- Concentration:Increasing the concentration of reactants generally increases the value of Kc and decreasing the concentration of products generally increases the value of Kc.
These factors can be used to manipulate the equilibrium position of a reaction according to Le Chatelier’s principle.
Kp = Kc for Ideal Gases, Select the reactions for which kp is equal to kc
For ideal gases, the relationship between Kp and Kc simplifies to:
Kp = Kc
This is because the partial pressure of an ideal gas is directly proportional to its concentration.
The mathematical derivation of this relationship is as follows:
Kp = (PNH3)^2 / (PN2)(PH2)^3
Substituting the ideal gas law into this equation, we get:
Kp = ([NH3]^2 / RT)^2 / ([N2] / RT)([H2] / RT)^3
Simplifying this equation, we get:
Kp = Kc(RT)^(Δn)
For ideal gases, Δn = 0, so we get:
Kp = Kc
Applications of Kp and Kc
Equilibrium constants are used in a variety of applications, including:
- Predicting the direction of reactions:The value of Kp or Kc can be used to predict whether a reaction will proceed in the forward or reverse direction under given conditions.
- Calculating equilibrium concentrations:The values of Kp or Kc can be used to calculate the equilibrium concentrations of reactants and products.
- Industrial processes:Equilibrium constants are used to optimize industrial processes, such as the production of ammonia and sulfuric acid.
- Environmental chemistry:Equilibrium constants are used to study the behavior of pollutants in the environment.
Detailed FAQs: Select The Reactions For Which Kp Is Equal To Kc
What is the significance of Kp and Kc?
Kp and Kc are equilibrium constants that provide insights into the extent of reactions in gas and solution phases, respectively.
How are Kp and Kc related?
For ideal gases, Kp and Kc are equal due to the direct proportionality between partial pressure and concentration.
What factors can affect Kp and Kc?
Temperature, pressure, and concentration can influence the values of Kp and Kc, as described by Le Chatelier’s principle.