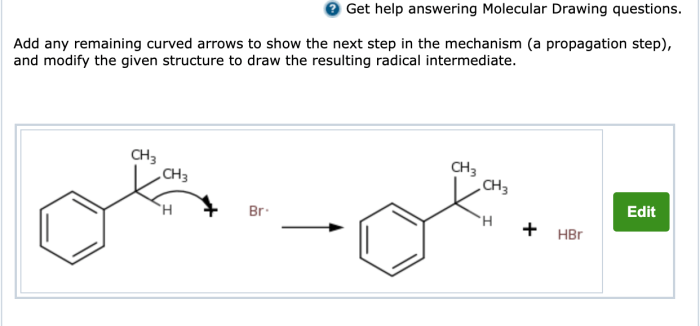
Add any remaining curved arrows to delve into the fascinating world of organic chemistry, where curved arrows serve as a visual guide to unravel the intricate dance of electrons and reveal the mechanisms behind chemical reactions.
Curved arrows are not mere embellishments but powerful tools that illuminate the hidden pathways of electron movement, providing a deeper understanding of how molecules interact and transform.
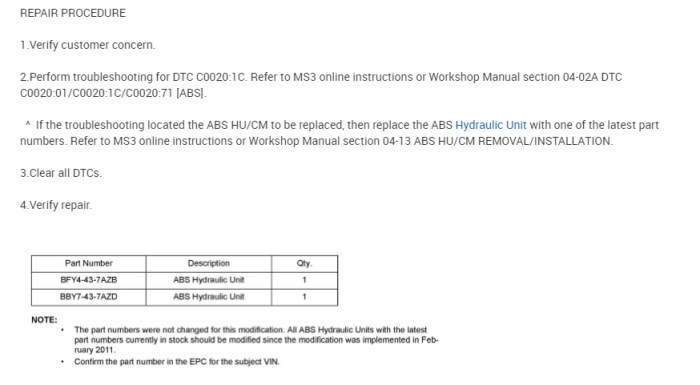
Introduction
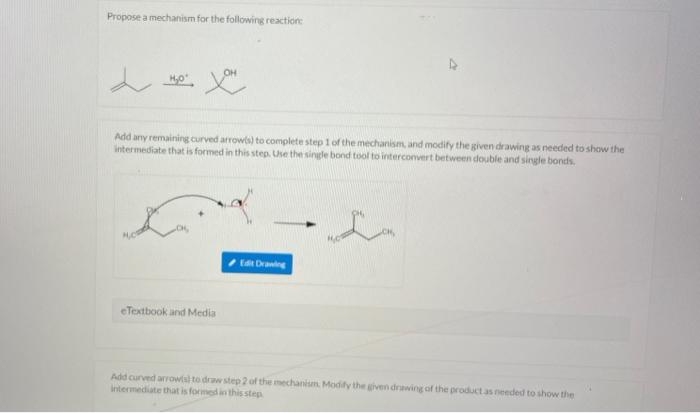
Curved arrows are an essential tool in organic chemistry for depicting the movement of electrons during a reaction. They provide a visual representation of the changes in the electronic structure of the reactants and products.
Curved arrows are used to show the flow of electrons from one atom or molecule to another. The head of the arrow points to the atom or molecule that is gaining electrons, while the tail points to the atom or molecule that is losing electrons.
As you continue adding any remaining curved arrows, you may want to refer to the FFA Floriculture Plant ID List for additional plant identification assistance. This comprehensive list provides detailed descriptions and images of various floriculture plants, making it a valuable resource for any gardener or plant enthusiast.
Examples of Reactions Where Curved Arrows Are Used
Curved arrows are used in a variety of organic chemistry reactions, including:
- Substitution reactions
- Addition reactions
- Elimination reactions
- Rearrangement reactions
Curved Arrows and Electron Movement
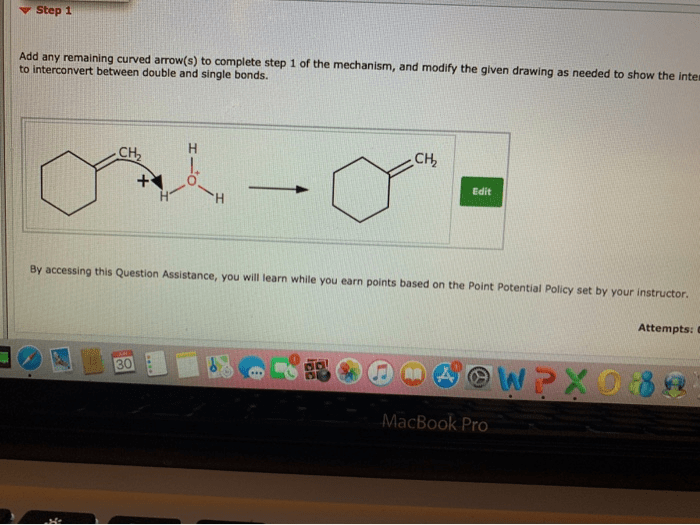
Curved arrows are a crucial tool in organic chemistry, used to depict the movement of electrons during chemical reactions. They help visualize the flow of electrons, making it easier to understand the mechanisms and outcomes of various reactions.
Rules for Drawing Curved Arrows
Drawing curved arrows follows specific rules to ensure clarity and accuracy:
- Origin and Destination:The tail of the arrow indicates the electron’s origin, while the head points to its destination.
- Electron Flow:Arrows always represent the movement of electrons from an area of high electron density to an area of low electron density.
- Partial Charges:Curved arrows can show the formation or removal of partial charges, denoted by the symbols δ+ and δ-.
- Resonance:Curved arrows can depict the movement of electrons in resonance structures, showing the delocalization of electrons.
- Curly Arrows:Curly arrows are used to indicate the movement of electrons in cyclic structures, such as benzene rings.
Adding Remaining Curved Arrows
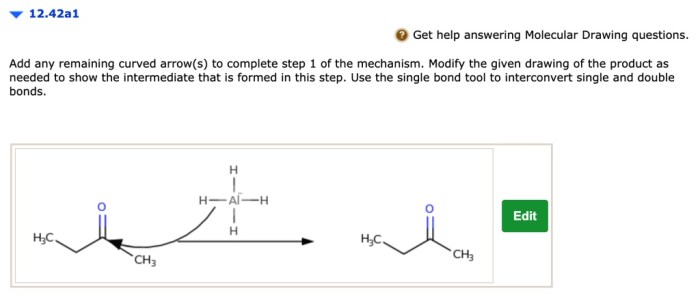
Adding remaining curved arrows is essential for completing the mechanism of a reaction and accurately representing the electron movement. It ensures that all electrons involved in the reaction are accounted for and that the reaction is balanced.
Step-by-Step Procedure for Adding Remaining Curved Arrows, Add any remaining curved arrows
Follow these steps to add remaining curved arrows:
- Identify all atoms or groups of atoms that have changed oxidation states.
- For each change in oxidation state, add a curved arrow to show the movement of electrons that caused the change.
- Electrons move from areas of high electron density to areas of low electron density.
- Curved arrows should start at a lone pair, a bond, or a negative charge and end at an empty orbital, a bond, or a positive charge.
- Ensure that the number of electrons entering a species equals the number of electrons leaving it.
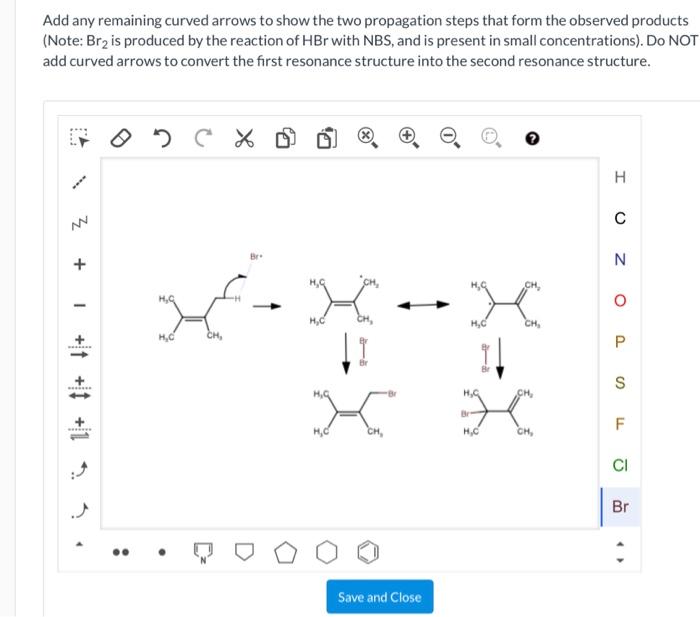
Example
Consider the following reaction:
“`CH 3CH 2OH + HBr → CH 3CH 2Br + H 2O“`
The following curved arrows show the electron movement:

Resonance Structures and Curved Arrows: Add Any Remaining Curved Arrows
Curved arrows are useful for depicting resonance structures, which are multiple Lewis structures that represent the same molecule or ion. Resonance structures differ only in the placement of electrons, not in the arrangement of atoms. The curved arrows show the movement of electrons as the molecule or ion resonates between its different structures.
Examples of Resonance Structures and Curved Arrow Representations
- Benzene: Benzene has six resonance structures, each with a different arrangement of double and single bonds. The curved arrows show the movement of electrons as the molecule resonates between these structures.
- Carbonate ion: The carbonate ion has three resonance structures, each with a different arrangement of double and single bonds. The curved arrows show the movement of electrons as the ion resonates between these structures.
Applications of Curved Arrows
Curved arrows are a powerful tool for visualizing and understanding the movement of electrons in organic reactions. They are used to show the flow of electrons from one atom or molecule to another, and can be used to explain a wide variety of reaction mechanisms.
One of the most important applications of curved arrows is in the prediction of product formation. By following the movement of electrons, it is possible to determine which products are likely to be formed in a given reaction. This information can be used to design new synthetic methods and to optimize existing ones.
Applications in Organic Chemistry
- Predicting product formation
- Understanding reaction mechanisms
- Designing new synthetic methods
- Optimizing existing synthetic methods
Answers to Common Questions
What is the significance of curved arrows in organic chemistry?
Curved arrows represent the movement of electrons, providing a visual representation of the changes in electron distribution during chemical reactions.
How do I determine the direction of curved arrows?
Curved arrows follow the rules of electron flow, moving from areas of high electron density to areas of low electron density.
Can curved arrows be used to represent resonance structures?
Yes, curved arrows can be used to depict the movement of electrons between resonance structures, showing the different possible electron distributions within a molecule.